ellume·lab is a versatile, handheld digital device designed for healthcare professionals at the point-of-care. ellume·lab offers a growing range of highly accurate, rapid diagnostic tests for the detection of common infectious diseases and provides practitioners with a suite of digital tools to support clinical decision-making and enhance patient engagement.
Learn More
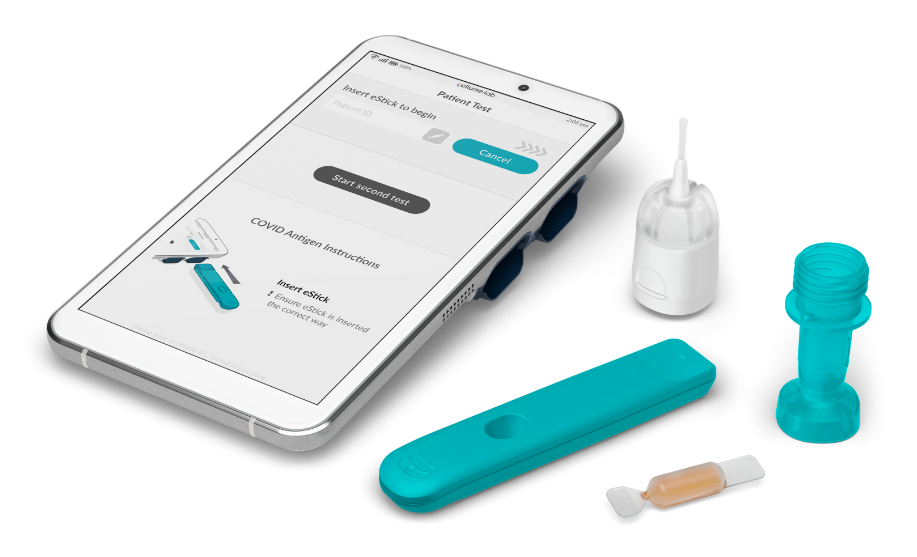
Highly accurate diagnostic tests
The ellume·lab diagnostic test range includes qualitative immunoassay tests for the detection of common infectious diseases and biological markers.
Two tests can be performed at the same time, with simple, animated on-screen instructions and rapid results in 15 minutes.
The COVID Antigen test (for the detection of active COVID-19 infections) has received U.S. FDA Emergency Use Authorization*. The COVID Serology test (for the detection of total antibodies to SARS-CoV-2) is pending regulatory clearance. Further tests, including a Flu A+B and a Flu A+B / RSV test, have undergone clinical studies and are being prepared for launch.
Learn More
Digital tools
ellume·lab provides practitioners with a suite of digital tools to support clinical decision-making and enhance patient engagement, on the same device. All ellume·lab digital tools offer secure, HIPAA compliant cloud connectivity for sharing results with patients or other healthcare professionals.
The tools have been designed by doctors for intuitive and efficient clinician use, with patient needs in mind. Digital tools include a BMI and growth calculator, dosage calculator, clinical camera and gallery, and medical diagrams.
